OVERVIEW
This Quickguide explains how to enroll as a Signatory Institution with the CIRB. Enrollment occurs in two parts: Part 1 establishes the institutional framework, and Part 2 establishes local context. Part 1 must be completed before progressing to Part 2.
BEFORE YOU BEGIN
1. Verify that all institutions enrolling into the CIRB have a Cancer Therapy Evaluation Program (CTEP) Site Code.
NOTE: Veterans Administration (VA) medical centers must contact the Office of Research and Development prior to beginning the enrollment process with the CIRB.
2. Confirm that all institutions are affiliated with a Network Group. The current list of supported Networks includes:
- NCI National Clinical Trials Network (NCTN)
- NCI Experimental Therapeutics Clinical Trials Network (ETCTN)
- NCI Community Oncology Research Program (NCORP)
- Phase 0/I/II Cancer Prevention Clinical Trials Program (Consortia) and Cancer Prevention Clinical Trials Network (CP-CTNet)
- Adult Brain Tumor Consortium (ABTC)
- Pediatric Brain Tumor Consortium (PBTC)
- AIDS Malignancy Consortium (AMC)
- Cancer Screening Research Network (CSRN)
STEPS

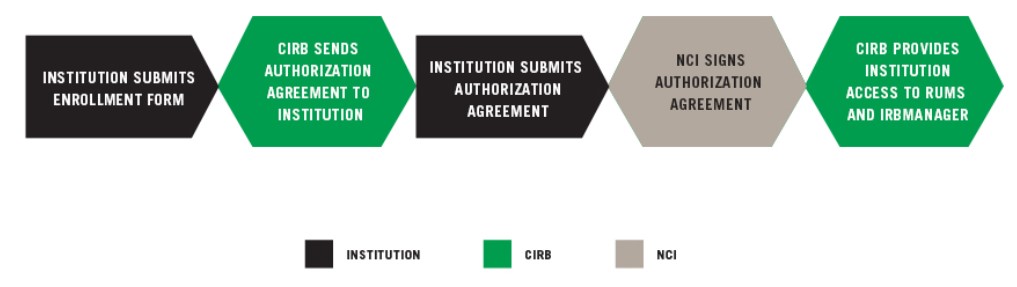
INSTITUTION SUBMITS ENROLLMENT FORM
The Enrollment Form:
Provides the CIRB with basic information about the Signatory Institution.
- Identifies the Signatory Institution Primary Contact.
- Identifies the institutional contact(s) who will view, manage, and edit the Roster Maintenance Update System (RUMS).
- If you are a CP-CTNet site, go to Navigating The CIRB For Consortia Sites And Cancer Prevention Clinical Trial Network (CP-CTNet) Organizations for information on how processes vary for you.
This form must be completed and emailed to CIRB Helpdesk.

CIRB SENDS AUTHORIZATION AGREEMENT TO INSTITUTION
The Authorization Agreement is between the NCI CIRB and Signatory Institution and outlines the division of responsibilities. The CIRB will send the Authorization Agreement to the Signatory Official identified on the Enrollment Form for electronic signature via DocuSign. Please let the official know to expect the email from Docusign. A sample, non-executable version of the Authorization Agreement available for your review.

INSTITUTION SUBMITS AUTHORIZATION AGREEMENT
The NCI reviews and signs the Authorization Agreement electronically and a fully executed copy is sent to the Signatory Official and all identified Signatory Institution staff. The NCI CIRB will then provide you with information regarding the next steps.

NCI SIGNS AUTHORIZATION AGREEMENT
The NCI reviews and signs the Authorization Agreement electronically and a fully executed copy is sent to the Signatory Official and all Signatory Institution Primary Contacts.

CIRB PROVIDES INSTITUTION ACCESS TO RUMS AND IRBManager
The CIRB Operations Office provides individuals identified on the Enrollment Form access to the CIRB roster. For more information, go to Updating Your CIRB Person Roster using RUMS and Updating Your CIRB Institution Roster using RUMS.
If you are at a Consortia or CP-CTNet site, go to Navigating The CIRB For Consortia Sites And Cancer Prevention Clinical Trial Network (CP-CTNet) Organizations for information on how processes vary for you.
To facilitate roster updates, you should ensure any PI that will be participating in the CIRB has the four CIRB registration numbers added to their 1572 in RCR. The four IRB registration numbers for the CIRB are:
- Adult Early Phase CIRB - IRB00009430
- Adult Late Phase CIRB - IRB00000781
- Pediatric CIRB - IRB00004296
- Cancer Prevention & Control CIRB - IRB00010018