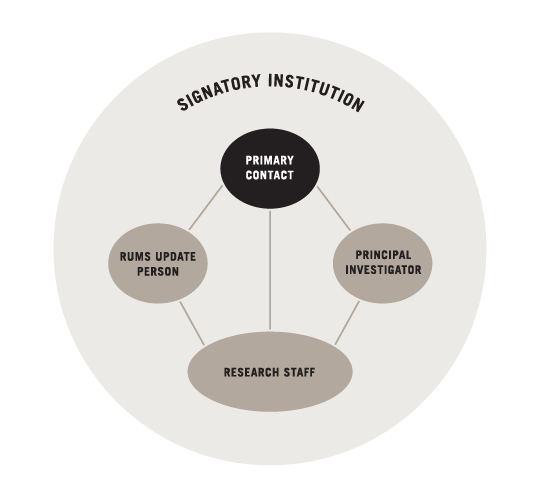
This Quickguide describes the four important roles at Institutions using the CIRB as their IRB of record.
SIGNATORY INSTITUTION PRIMARY CONTACT
A Signatory Institution Primary Contact:
- Is the individual at the Signatory Institution who acts as the point of contact for the CIRB.
- Is the individual to be contacted by the CIRB for questions about the research conducted at the Signatory Institution, Component Institution(s), or Affiliate Institution(s).
- Receives or is copied on all correspondence from the CIRB to the Signatory Institution and the Signatory Institution Principal Investigator (PI) or Investigators.
- Is responsible for submitting the Annual Signatory Institution Worksheet, and may assist with the completion of other Worksheets.
Note: A Signatory Institution may have more than one Signatory Institution Primary Contact, if needed.
RUMS UPDATE PERSON
A RUMS Update Person:
- Receives administrative rights to the CTSU’s Roster Update Maintenance System (RUMS).
- Uses the RUMS interface to view, manage, and make changes to their own Signatory Institution on the CIRB Roster.
- For more information, go to Updating Your CIRB Person Roster.
- If you are an NCI Division of Cancer Prevention (DCP) Consortia or CP-CTNet site, go to Navigating The CIRB For NCI Division of Cancer Prevention Consortia Sites And Cancer Prevention Clinical Trial Network (CP-CTNet) Organizations for information on how processes vary for you.
PRINCIPAL INVESTIGATOR
A PI:
- Is “employed by” or "has a relationship with" the Signatory Institution.
- Is a member of the group coordinating the study, and is able to open studies within the Network.
The PI is responsible for the research at their Institution and all research activities conducted by the research staff. This includes any research activity at Component or Affiliate Institutions for all studies opened in the PI’s name.
RESEARCH STAFF
Research Staff:
- Needs access to the IRBManager to complete the various required Worksheets on behalf of the Signatory Institution or PIs.
USEFUL TIPS
- Anyone who works with the CIRB must have an active CTEP Person ID. To register or update information with the Cancer Therapy Evaluation Program-Identity and Access Management, go to the directions section.
- Sub-Investigators and other support staff who do not require IRBManager access will be able to use the CTSU website through their associations on the Network rosters.