OVERVIEW
BEFORE YOU BEGIN
- The Network announces to participating institutions that the study is active and institutions may proceed with opening it.
- Your institution’s Annual Signatory Institution Worksheet has been approved by the CIRB. For more information, go to Establishing Your Signatory Institution.
- The Annual Principal Investigator Worksheet for the PI running the study at your institution has been approved by the CIRB. For more information, go to Establishing a Principal Investigator.
STEPS

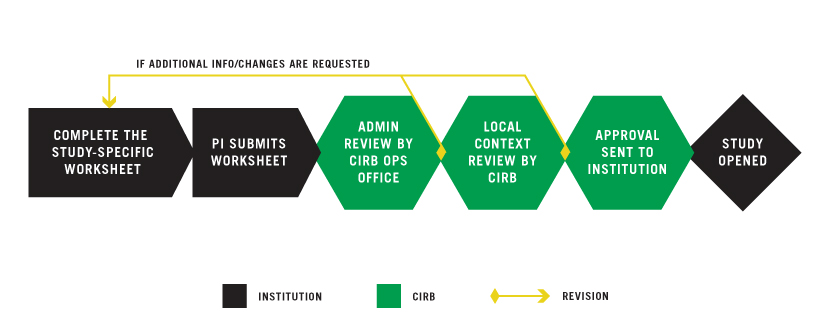
PI OR DESIGNEE COMPLETES THE STUDY-SPECIFIC WORKSHEET
The Study-Specific Worksheet serves as your institution’s request to open a study overseen by the NCI CIRB. This Worksheet can be completed in IRBManager by either the PI or a designated member of the institution’s research staff; however, only the PI can submit it. The purpose of the Worksheet is to verify that the information previously approved for the institution and the PI still applies to a specific study.
The CIRB expects that the Study-Specific Worksheet will only include minor changes to the previously approved research processes outlined in the institutional and PI Worksheets, thus allowing it to go through an expedited review. Minor changes to your institutional boilerplate language which address study-specific variations are allowed to be submitted when opening a study.
Any substantial changes (addition of risks to the consent form, addition of a new consent form, other changes to the study) are not eligible for expedited review. Substantial changes require an amendment to the study protocol. Contact the Study Chair and Network with any substantial changes.

PI SUBMITS WORKSHEET
If someone other than the PI completes the Study-Specific Worksheet, IRBManager will automatically generate an email to the PI requesting review, confirm intent to comply, and submission of the Worksheet in IRBManager. Confirming intent to comply and submitting the Worksheet is a multi-step process. The PI must click the submit button on the final page or the form will NOT be submitted. For more information, go to Starting a New Worksheet in IRBManager.

CIRB OPERATIONS OFFICE CONDUCTS ADMINISTRATIVE REVIEW
After the PI approves and submits the Worksheet, IRBManager routes it to the CIRB Operations Office for administrative review. This process typically takes two days. The CIRB Operations Office may request additional information from the submitter. The submitter responds by updating the Worksheet beginning at Step 1.

CIRB LOCAL CONTEXT COMMITTEE CONDUCTS REVIEW
Once the administrative review is complete, IRBManager routes the Worksheet to the CIRB Local Context Subcommittee for review. This review typically takes two days. If the CIRB Local Context Subcommittee requests additional information, you will receive an email notifying you that the Worksheet has been returned to the institution. The institution should then update the Worksheet beginning at Step 1.

APPROVAL TO INSTITUTION
Once the CIRB Local Context Subcommittee approves the Worksheet, the CIRB Operations Office generates the Approval Letter and sends it to:
- the PI
- the person submitting the Worksheet, and
- the Signatory Institution Primary Contact(s).

STUDY OPENED AT INSTITUTION
Once you have received the Approval Letter, the study may be opened at your institution.