OVERVIEW
This Quickguide describes Signatory Institutions, Component Institutions, and Affiliate Institutions.
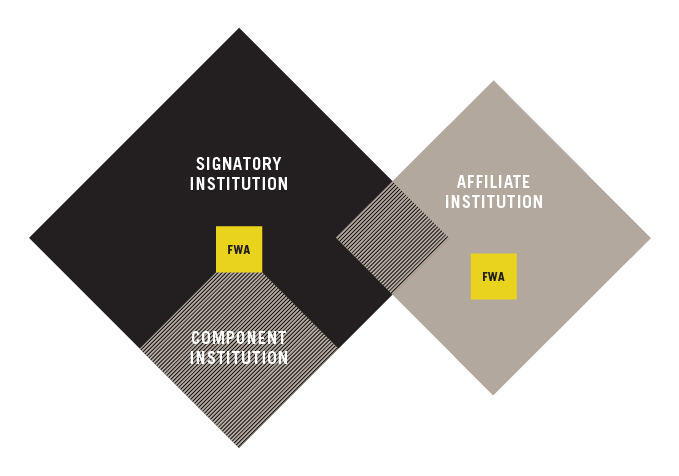
SIGNATORY INSTITUTION
A Signatory Institution:
- Has a direct relationship with the CIRB.
- Signs the Authorization Agreement and the Division of Responsibilities document.
- Has the option of including Affiliate and Component Institutions.
- Is responsible for defining the local context considerations and reporting to the CIRB using the Annual Signatory Institution Worksheet.
- Ensures that all covered entities conform to the local context considerations.
NOTE: The responsibilities of the Signatory Institution are listed on the Authorization Agreement.
COMPONENT INSTITUTION
A Component Institution:
- Operates under a different name than the Signatory Institution.
- Uses the same Federal-wide Assurance (FWA) number as the Signatory Institution.
- Adheres to the same local context considerations as the Signatory Institution. Uses the same boilerplate language and institutional requirements of the Signatory Institution.
- Conducts research that is monitored by the same office as the Signatory Institution.
NOTE: The Signatory Institution has legal authority over the Component Institution.
AFFILIATE INSTITUTION
An Affiliate Institution:
- Has a direct relationship with the Signatory Institution and its own FWA number.
- Adheres to the same local context considerations of the Signatory Institution.
- Uses the same boilerplate language and institutional requirements of the Signatory Institution.
- Conducts research that is monitored by the same office as the Signatory Institution.
USEFUL TIPS
All institutions where CTEP-sponsored clinical research trials are conducted must have a unique CTEP Site Code. If you need a CTEP Site Code or have questions, go to the Enterprise Core Unit (ECU) at ecuhelpdesk@mail.nih.gov.