OVERVIEW
BEFORE YOU BEGIN
- The Network Group coordinating office staff receives a reminder email and access to the online continuing review application in IRBManager from the CIRB. The email and application indicates the study's expiration date and the application due date. This email serves as an important reminder to ensure the study's approval does not lapse. You can also download a copy of the CIRB Continuing Review Application.
STEPS

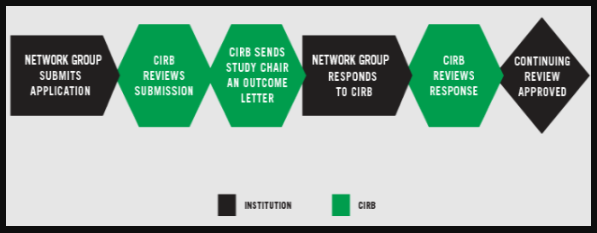
NETWORK GROUP COORDINATING STAFF SUBMITS APPLICATION
The Network Group coordinating office staff completes and submits the Continuing Review through IRBManager to the CIRB by the submission deadline, 14 days before the scheduled CIRB meeting.
A complete submission package includes the CIRB application, protocol, model consent form, and, if applicable, Investigator's Brochure, recruitment material, and patient reported outcomes.
The Coordinating Center should contact the designated CIRB Committee directly with questions about the application process.
- Adult CIRB- Late Phase Emphasis: adultcirb@emmes.com
- Adult CIRB- Early Phase Emphasis: earlyphasecirb@emmes.com
- Cancer Prevention and Control CIRB: CPCCIRB@emmes.com
- Pediatric CIRB: pediatriccirb@emmes.com

CIRB REVIEWS SUBMISSION
The CIRB Operations Office determines whether the continuing review qualifies for expedited review or if it requires full board review. Expedited reviews are sent to the CIRB Chair and full board reviews are reviewed at a convened meeting.

CIRB SENDS STUDY CHAIR AN OUTCOME LETTER
Within seven days following the meeting, the CIRB emails an Outcome Letter to the Study Chair and Network Group coordinating office staff. The possible outcomes are: Approved, Approved Pending Modifications, or Tabled. The letter states the board’s stipulations and recommendations for changes to the protocol.

NETWORK GROUP COORDINATING OFFICE STAFF RESPONDS TO CIRB
The Network Group coordinating office staff responds to the CIRB’s recommendations for study modifications by submitting the necessary revised documents within 14 days.

CIRB REVIEWS NETWORK GROUP COORDINATING OFFICE STAFF RESPONSE
The CIRB reviews the Network Group coordinating office staff's response via expedited review procedures or convened CIRB review. If convened review is required, the submission will be reviewed at the next CIRB meeting. At that point, an approval will be issued or additional modifications will be required. If additional modifications are required, the process repeats at step 3.

CIRB ISSUES APPROVAL
The CIRB issues an Approval Letter to the Study Chair and Network Group coordinating office staff within two days of the approval determination.