Part 2: Documenting Local Context
OVERVIEW
This instruction provides an overview of the process for enrolling as a Signatory Institution with the CIRB. Enrollment occurs in two parts: Part 1 establishes the institutional framework, and Part 2 establishes local context. Part 1 must be completed before progressing to Part 2.
STEPS

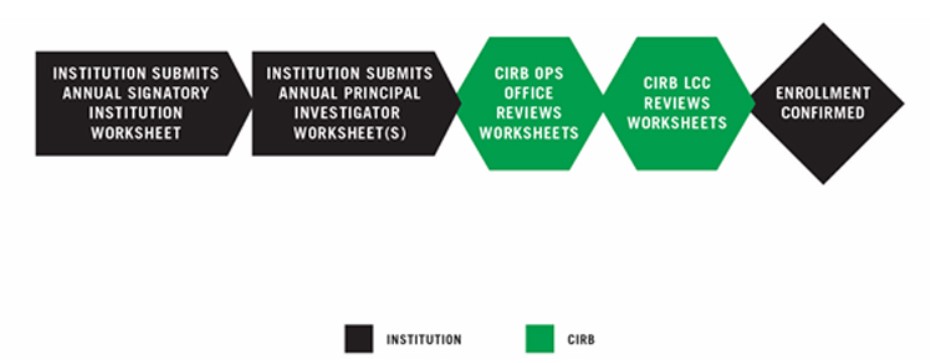
INSTITUTION SUBMITS ANNUAL SIGNATORY INSTITUTION WORKSHEET
The Annual Signatory Institution Worksheet provides the CIRB with information about the Signatory Institution’s legal, regulatory, and community context. It is completed and submitted to the CIRB using IRBManager. For more information, go to Establishing Your Signatory Institution.

INSTITUTION SUBMITS ANNUAL PRINCIPAL INVESTIGATOR WORKSHEET(S)
The Annual Principal Investigator Worksheet provides the CIRB with information about a Principal Investigator and local research, recruitment, and consent processes. To complete enrollment, one Annual Principal Investigator Worksheet must be submitted. PIs must have an approved Annual Principal Investigator Worksheet prior to opening a study. This Worksheet is completed by the PI or designee and submitted using IRBManager. For more information, go to Establishing a Principal Investigator.

CIRB OPERATIONS OFFICE REVIEW AND APPROVAL
-
The CIRB Operations Office reviews the Worksheet and works closely with the Signatory Institution to ensure all information is complete.
-
The completed Worksheet is routed to the CIRB Local Context Subcommittee for review and approval.
-
The CIRB Local Context Subcommittee member ensures all information is appropriate and accurate according the current regulations. If so, the member approves the Worksheet and emails an Approval Letter.

ENROLLMENT CONFIRMED
The CIRB emails a Requirements Complete Letter to the Signatory Institution Primary Contact and Principal Investigators confirming the Signatory Institution’s enrollment.
Note: Once enrollment is confirmed, update your Federalwide Assurance (FWA). Modify item #6 on the FWA form, if applicable. The IRB registration numbers for the CIRB are:
- Adult Early Phase CIRB - IRB00009430
- Adult Late Phase CIRB - IRB00000781
- Pediatric CIRB - IRB00004296
- Cancer Prevention & Control CIRB - IRB00010018