Submitting a Study for Continuing Review (ETCTN)
OVERVIEW
This Quickguide provides Study Chairs with an overview of the continuing review process.
BEFORE YOU BEGIN
- The Study Chair receives a reminder letter and a continuing review application from the CIRB. The letter indicates the study's expiration date and the application due date. This letter serves as an important reminder to ensure the study's approval does not lapse. You can also download a copy of the CIRB Continuing Review Application.
STEPS

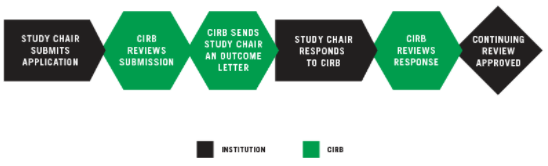
STUDY CHAIR SUBMITS APPLICATION
The Study Chair emails the completed application to the CIRB by the submission deadline, 14 days before the scheduled CIRB meeting.
A complete submission package includes the CIRB application, protocol, model consent form, and, if applicable, Investigator's Brochure, recruitment material, and patient reported outcomes.

CIRB REVIEWS SUBMISSION
The CIRB Operations Office determines whether the continuing review qualifies for expedited review or if it requires full board review. Expedited reviews are sent to the CIRB Chair and full board reviews are reviewed at a convened meeting.

CIRB SENDS STUDY CHAIR AN OUTCOME LETTER
Within seven days following the meeting, the CIRB emails an Outcome Letter to the Study Chair. The possible outcomes are: Approved, Approved Pending Modifications, or Tabled. The letter states the board’s stipulations and recommendations for changes to the protocol.

STUDY CHAIR RESPONDS TO CIRB
The Study Chair responds to the CIRB’s recommendations for study modifications by submitting the necessary revised documents within 14 days.

CIRB REVIEWS STUDY CHAIR’S RESPONSE
The CIRB reviews the Study Chair’s response via expedited review procedures or convened CIRB review. If convened review is required, the submission will be reviewed at the next CIRB meeting. At that point, an approval will be issued or additional modifications will be required. If additional modifications are required, the process repeats at step 3.

CIRB ISSUES APPROVAL
The CIRB issues an Approval Letter to the Study Chair within two days of the approval determination.